Silicon Crystals Are Semiconductors Which of the Following
Correct option is D Phosphorous which is pentavalent produces n-type semiconductor. Silicon crystals have less free electrons at room temperature to germanium crystals.
Semiconductor Material Silicon Semiconductor Shindengen Electric Mfg Co Ltd
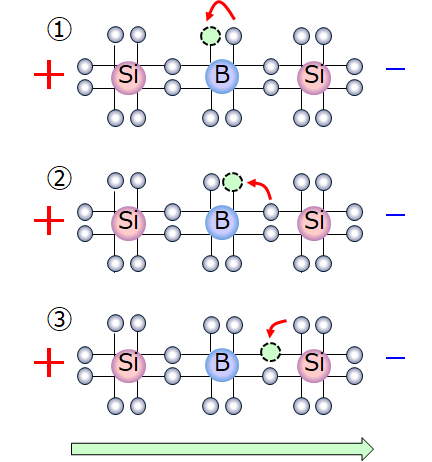
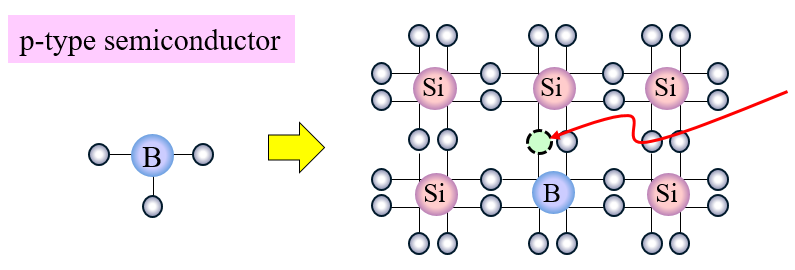
Boron doped with silicon.

. The valence of an impurity added to germanium crystal in order to convert it into a P-type semiconductor is asked May 25 2019 in Physics by BrijeshSarangi. NoteAn intrinsicpure semiconductor is indeed a pure semiconductor without any noticeable dopant species present sometimes termed an undoped. Of mentioned elements which of following is a semiconductor.
If you are looking for a reviewer in Electronics Engineering this will definitely help. Materials Devices And Simple Circuits with-. Question 19 Which of the following elements when doped into silicon would yield an n-type semiconductor.
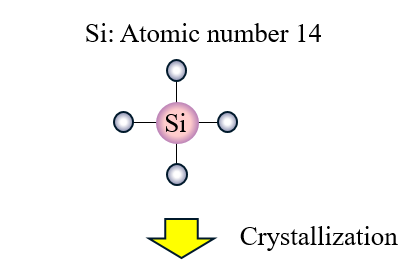
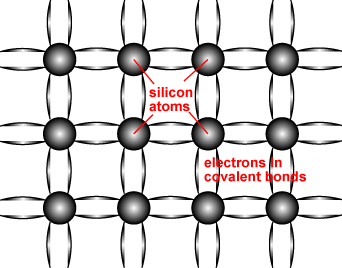
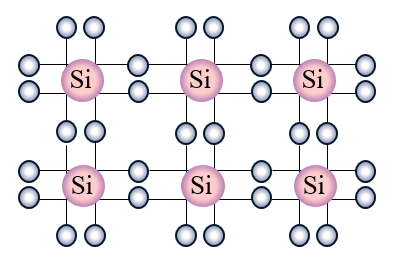
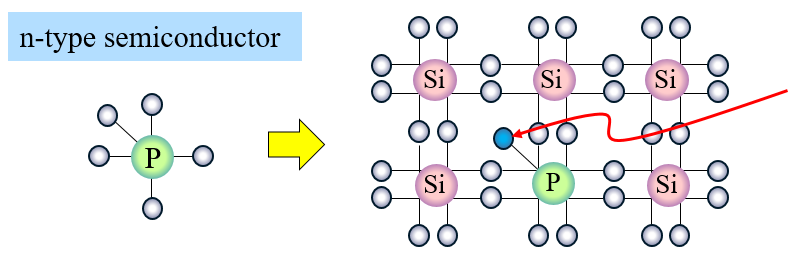
When we replace a few of these Si atoms with P atoms which have a valency of 5 4 out of these 5 outermost electrons will be bonded with the surrounding Si atoms. Thus in silicon crystals each Si atom is connected to 4 different Si atoms. Whereas when a germanium or silicon semiconductor is doped with a group 15 element then a n-type semiconductor having more.
Semiconductors from the book Electronic Principles 7th Edition by Albert Malvino. Here only four electrons of phosphorous are used in making bonds with silicon crystal. Germanium selenium O phosphorus O gallium O carbon.
Germanium is a group 14 element hence it cannot be doped with Si to improve its conductivity. The p-n junction diodes consist of two adjacent p and n semiconductor elements. Good examples of semiconductor materials are germanium selenium and silicon.
An extrinsic semiconductor which has been doped with electron donor atoms is called an n-type semiconductor because the majority of charge carriers in the crystal are negative. This means the silicon would have a much lower current cut off from the collector than germanium. The most commonly used semiconductor materials are silicon germanium and gallium arsenide.
P atoms introduce additional mobile negative charges. Silicon atoms have a valency of 4 this means that there are 4 electrons in their outermost orbital. The fifth electron remains free.
Hence the correct options are A and B. These crystals are more difficult to grow than silicon. Of mentioned elements which of following is a semiconductor.
In the past compound semiconductors were not used in the widespread commercial applications and high production volumes typical for silicon. However after 1990 a few semiconductor devices using organic semiconductors and semiconducting polymers have been developed signaling the birth of a futuristic technology of polymer-electronics and molecular-electronics. Compound semiconductors also tend to be more fragile.
Doping Aluminum with Silicon forms a p-type semiconductor because Silicon is a 14 group element and Aluminum is a 13 group element. The p and n materials are simply semiconductors such as silicon Si or germanium Ge with atomic contamination. Some semiconductor devices are junction diodes a 2-electrode device and bipolar junction transistor a 3-electrode device.
Silicon has an extremely stable structure and is an effective thermal conductor which is why it is widely applied in the manufacture of integrated circuits ICs and nanoelectronics. Question 19 Which of the following elements when doped into silicon would yield an n-type semiconductor. Nitrogen Phosphorous Bismuth are 15 group elements.
- A Low stable - B Can be operated only at low temperatures. P atoms introduce additional mobile positive charges. Germanium selenium O phosphorus O gallium O carbon MacBook Pro.
The elemental semiconductors are those composed of single species of atoms such as silicon Si germanium Ge and tin Sn in column IV and selenium Se and tellurium Te in column VI of the periodic. TDPAC measurements were carried out under external magnetic fields with strengths of 048 T. This is the Multiple Choice Questions in Chapter 2.
The type of impurity present determines the type of. When a germanium or silicon semiconductor is doped with a group 13 element then a p-type semiconductor having more number of holes as majority carriers is formed. Semiconductors such as germanium Ge silicon Si gallium arsenide GaAs and indium phosphide InP are widely used in electronics and optics.
The most popular and common semiconductor material is. Which of the following is a correct reason for the increase in the conductivity of Si crystals when a small fraction of Si atoms are replaced with those of a different element. Polycrystalline silicon poly Si silicon dioxide SiO 2 both doped and.
It is either doped with group 13 element B Al Ga In to make a p type semiconductor or it is doped with a group 15 element N P As Sb to make n type semiconductor. The doping agents used are of two types resulting in two types of extrinsic semiconductor. CVD can be used to deposit many materials but in silicon semiconductor processing the materials generally encountered in addition to epitaxial silicon are.
This study reports on the local exploration of magnetic field effects in semiconductors including silicon Si germanium Ge gallium arsenide GaAs and indium phosphide InP using the time differential perturbed angular correlation TDPAC technique. The most popular and common semiconductor material is. In Preparation for the ECE Board Exam make sure to expose yourself and familiarize in each and every questions compiled here taken from various sources including but not limited to past Board Exam Questions in.
The correct answer is Germanium. The semiconductor is a class of crystalline solids intermediate in electrical conductivity between a conductor and an insulator. Which of the following is the disadvantage of silicon semiconductor detector.
The number of defects in the crystal is higher and the cost of making the crystal is higher. Silicon crystals are semiconductors. So they will give n-type semiconductors.
Si is group 14 element. Solve any question of Semiconductor Electronics. This is the Multiples Choice Questions Part 6 of the Series in Solid State DevicesCircuits as one of the Electronics Engineering topic.
Semiconductor Material Silicon Semiconductor Shindengen Electric Mfg Co Ltd
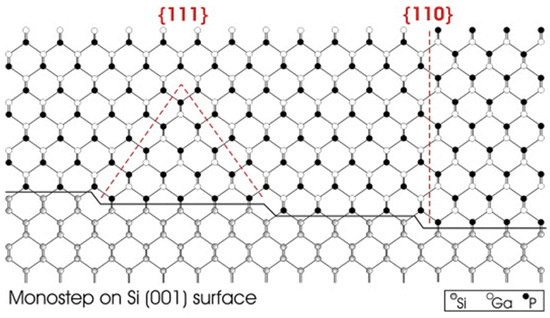
Crystals Free Full Text Heteroepitaxial Growth Of Iii V Semiconductors On Silicon Html
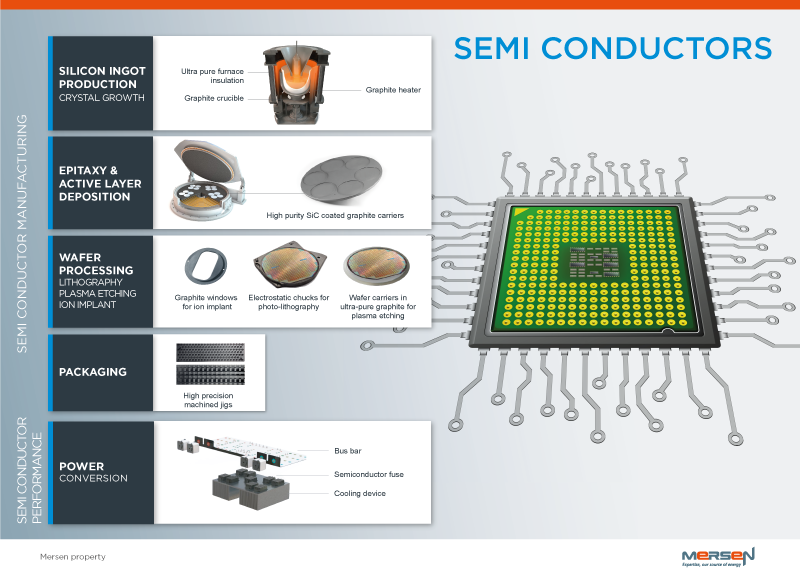
Mersen Semiconductor Silicon Ingot Carbon Felt Silicon Carbide
![]()
Semiconductor Device Electronics Britannica
Semiconductor Material Silicon Semiconductor Shindengen Electric Mfg Co Ltd

What Are The Major Reasons Behind Silicon Uses In Electronics
![]()
Pure Silicon Crystal Archives The Fact Factor
How Semiconductor Works Properties Types Uses Of Semiconductor

What Are The Major Reasons Behind Silicon Uses In Electronics

Semiconductor Basics Semiconductor Physics Tutorial
![]()
What Is Silicon And Why Are Computer Chips Made From It Extremetech
What Are Semiconductors Materials Science Engineering
What Are Semiconductors Materials Science Engineering
Semiconductor Structure Pveducation
New Form Of Silicon Advances Semiconductor Technology Materials Today
Semiconductor Material Silicon Semiconductor Shindengen Electric Mfg Co Ltd

Semiconductor Basics Semiconductor Physics Tutorial

Crystal Defects Enhancing Silicon Semiconductor Properties By
Semiconductor Material Silicon Semiconductor Shindengen Electric Mfg Co Ltd
Comments
Post a Comment